What is BBS?
Prevalence of BBS3,14,19
US
~4,000 to 5,000 individuals
Europe
~4,000 to 5,000 individuals
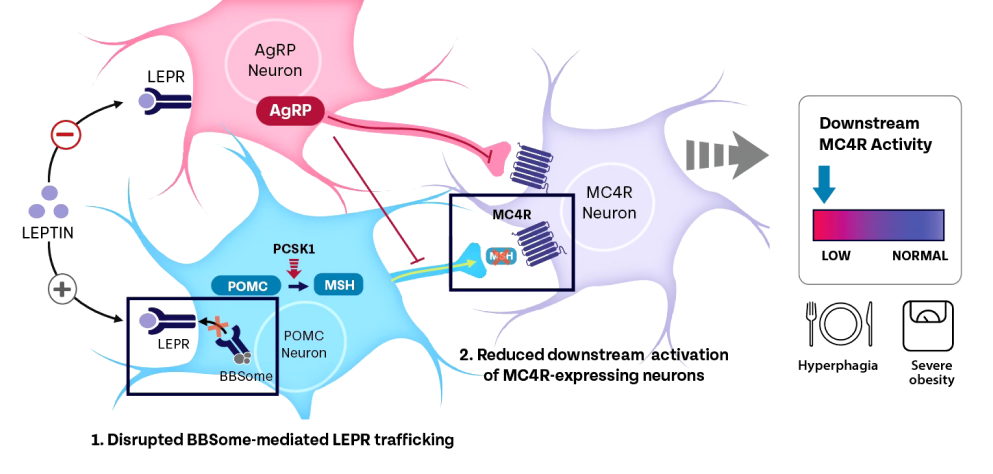
What is the relationship between MC4R pathway impairment and BBS?
AgRP, agouti-related peptide; BBS, Bardet-Biedl syndrome; BBSome, complex of 8 BBS proteins; LEPR, leptin receptor; MC4R, melanocortin-4 receptor; MSH, melanocyte-stimulating hormone; PCSK1, proprotein convertase subtilisin/kexin type 1; POMC, proopiomelanocortin.
AgRP, agouti-related peptide; BBS, Bardet-Biedl syndrome; BBSome, complex of 8 BBS proteins; LEPR, leptin receptor; MC4R, melanocortin-4 receptor; MSH, melanocyte-stimulating hormone; PCSK1, proprotein convertase subtilisin/kexin type 1; POMC, proopiomelanocortin.
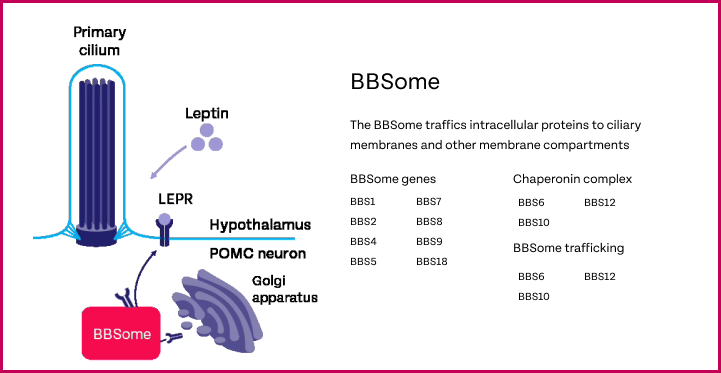
Genes associated with BBS
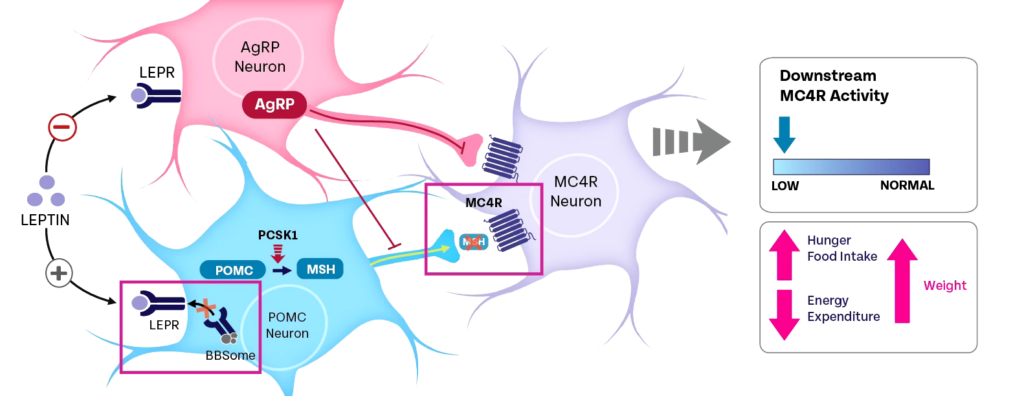
At least 28 genes have been associated with BBS to date.20 BBS genes play a critical role in MC4R signaling.17,20,21 Variants in genes encoding the BBSome (a complex of 8 BBS proteins), or those that support BBSome function, can result in dysfunction of primary cilium, leading to impaired leptin receptor trafficking. This impairs MC4R pathway signaling, thereby causing hyperphagia and early-onset, severe obesity.1,5-10,12,13,15,16,18,21,23,25,26
The scientific understanding of BBS is constantly evolving; therefore, novel genes may still be discovered to be associated with BBS in the future.7,8,20
Primary Cilia Play a Role in the Development of Obesity in BBS2,4,5,9-11,18,21,24
Discover more
Learn about ways to help diagnose patients with BBS, a rare genetic disease.
BBS, Bardet-Biedl syndrome; MC4R, melanocortin-4 receptor
References
- Castro-Sánchez S, et al. J Pediatr Genet. 2013;2(2):77-83.
- da Fonseca et al. J Diabetes Complications. 2017;31:1549-1561.
- Data on file, Rhythm Pharmaceuticals.
- Farooqi and O’Rahilly. Nat Clin Pract Endocrinol Metab. 2008;4:569-577.
- Feuillan et al. J Clin Endocrinol Metab. 2011;96:E528-E535.
- Focșa IO, et al. Biomed Rep. 2021;15(6):103.
- Forsyth R, Gunay-Aygun M. GeneReviews. Accessed February 21, 2024. https://www. ncbi.nlm.nih.gov/books/NBK1363
- Forsythe E, Beales PL. Eur J Hum Genet. 2013;21(1):8-13.
- Geets et al. Clin Genet. 2019;95:23-40.
- Guo et al. PLoS Genet. 2016;12:e1005890.
- Guo and Rahmouni. Trends Endocrinol Metab. 2011;22:286-293.
- Lee JE, Gleeson JG. Genome Med. 2011;3(9):59.
- Manara E, et al. Ital J Pediatr. 2019;45(1):72.
- National Organization for Rare Disorders. Accessed March 8, 2021. https://rarediseases.org/rare-diseases/bardet-biedl-syndrome/
- Nishimura Y, et al. Adv Sci (Weinh). 2018;6(1):1801138.
- Park SM, et al. Front Cell Neurosci. 2019;13:218.
- Seo et al. Hum Mol Genet. 2009;18:1323-1331.
- Seo et al. PLoS Genet. 2011;7(11):e1002358.
- Suspitsin EN, et al. Mol Syndromol. 2016;7(2):62-71.
- Tomlinson JW. Bardet-Biedl syndrome: A focus on genetics, mechanisms and metabolic dysfunction. Diabetes Obes Metab. 2024; 26(Suppl. 2): 13-24. doi:10.1111/dom.15480
- Vaisse et al. Cold Spring Harb Perspect Biol. 2017;9:a028217.
- Wang et al. J Clin Invest. 2021;131:e146287.
- Ware SM, et al. Proc Am Thorac Soc. 2011;8(5):444-450.
- Yazdi et al. PeerJ. 2015;3:e856.
- Zacchia M, et al. Kidney Dis (Basel). 2017;3(2):57-65.
- 26. Zuo X, et al. J Biol Chem. 2019;294(17):6710-6718.
